Detect More Variants that Matter in Cancer
The promise of personalized medicine depends on discovering critical insights into tumor biology that can uncover new therapeutic targets and biomarker signatures that inform care. Structural variants (SVs) are a hallmark of cancer, yet current cytogenetic and molecular methods fail to detect all classes and sizes of SVs, missing a significant amount of information critical to understanding cancer biology.1-16 Studies have shown that a significant portion of SVs detected by optical genome mapping (OGM) are being missed by traditional cytogenetic and next-generation sequencing (NGS) approaches.2,16,17
Leverage OGM to maximize SV detection, so you can build comprehensive genomic profiles of hematological malignancies and solid tumors, deepening your understanding of cancer biology and unlocking new precision medicine possibilities.

Maximize pathogenic and actionable findings.
Leverage OGM’s unbiased, genome-wide, and high-resolution capabilities to detect all classes of SVs, maximize pathogenic findings and increase the number of informed cases in hematological malignancies and solid tumor samples.
Discover new cancer biomarkers.
Unveil new meaningful events with OGM, from single SVs to complex events such as chromothripsis, or even genome-wide signatures such as homologous recombination deficiency.

Better characterize cancer samples.
Combine findings from OGM and NGS to uncover more pathogenic insights and generate comprehensive genomic profiles of tumor samples that deepen the understanding of underlying pathology.

Read Our Case Studies
Explore these cancer genomic case studies and learn how OGM uncovers SVs that short- and long-read sequencing can’t reliably identify.
Read MoreApplications
Hematological Malignancies
Detect chromosomal aberrations commonly found with traditional cytogenetics while revealing incremental pathogenic findings using a single, easy-to-implement OGM workflow.
Learn MoreSolid Tumors
Use OGM to uncover meaningful SVs in solid tumor genomes that can’t be seen with NGS approaches.
Learn MoreDiscover How Bionano Solutions Benefit Clinical Cancer Research

Check out expert presentations on the utility of OGM for evaluating hematological malignancies.
Watch Videos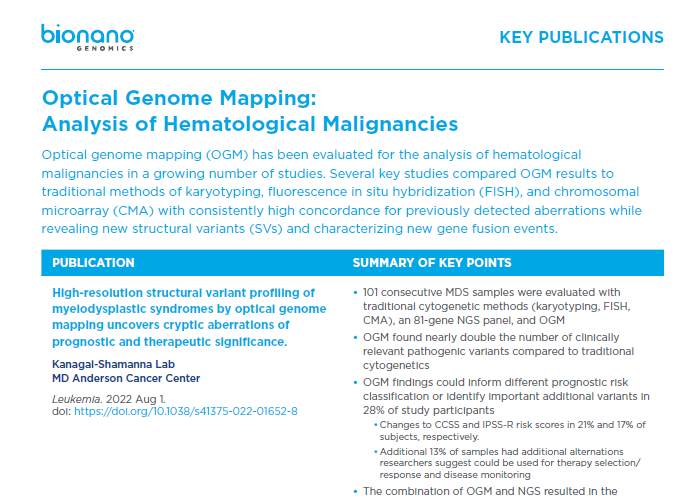
Explore the growing evidence for OGM performance in heme in this downloadable publication summary.
Read More
Learn how researchers are using OGM to better characterize and discover SVs across multiple solid tumor types.
Watch VideosLearn more about OGM
Read about what structural variations are and why they matter.
Learn MoreSee how OGM reveals structural variation in a way that’s never been done before.
Learn MoreFind the latest research in our Publications Library.
Learn More
Sign up to get the latest updates on Bionano solutions for oncology applications.
Sign Me UpRelated Focus Areas & Applications
- Balducci E, Kaltenbach S, Villarese P, et al. Optical genome mapping refines cytogenetic diagnostics, prognostic stratification and provides new molecular insights in adult MDS/AML patients. Blood Cancer J. 2022;12(9):126. Published 2022 Sep 2. doi:10.1038/s41408-022-00718-1
- Bayard Q, Cordier P, Péneau C, et al. Structure, Dynamics, and Impact of Replication Stress-Induced Structural Variants in Hepatocellular Carcinoma. Cancer Res. 2022;82(8):1470-1481. doi:10.1158/0008-5472.CAN-21-3665
- Gerding WM, Tembrink M, Nilius-Eliliwi V, et al. Optical genome mapping reveals additional prognostic information compared to conventional cytogenetics in AML/MDS patients. Int J Cancer. 2022;150(12):1998-2011. doi:10.1002/ijc.33942
- Kriegova E, Fillerova R, Minarik J, et al. Whole-genome optical mapping of bone-marrow myeloma cells reveals association of extramedullary multiple myeloma with chromosome 1 abnormalities. Sci Rep. 2021;11(1):14671. Published 2021 Jul 19. doi:10.1038/s41598-021-93835-z
- Levy B, Baughn L, Chartrand S, et. al. A national multicenter evaluation of the clinical utility of optical genome mapping for assessment of genomic aberrations in acute myeloid leukemia. medRxiv. 2020 Nov 10. doi: https://doi.org/10.1101/2020.11.07.20227728
- Lühmann JL, Stelter M, Wolter M, et al. The Clinical Utility of Optical Genome Mapping for the Assessment of Genomic Aberrations in Acute Lymphoblastic Leukemia. Cancers (Basel). 2021;13(17):4388. Published 2021 Aug 30. doi:10.3390/cancers13174388
- Neveling K, Mantere T, Vermeulen S, et al. Next-generation cytogenetics: Comprehensive assessment of 52 hematological malignancy genomes by optical genome mapping. Am J Hum Genet. 2021;108(8):1423-1435. doi:10.1016/j.ajhg.2021.06.001
- Nimer SD. Is it important to decipher the heterogeneity of “normal karyotype AML”?. Best Pract Res Clin Haematol. 2008;21(1):43-52. doi:10.1016/j.beha.2007.11.010
- Rack K, De Bie J, Ameye G, et al. Optimizing the diagnostic workflow for acute lymphoblastic leukemia by optical genome mapping [published correction appears in Am J Hematol. 2022 May 10;:]. Am J Hematol. 2022;97(5):548-561. doi:10.1002/ajh.26487
- Sabatella M, Mantere T, Waanders E, et al. Optical genome mapping identifies a germline retrotransposon insertion in SMARCB1 in two siblings with atypical teratoid rhabdoid tumors. J Pathol. 2021;255(2):202-211. doi:10.1002/path.5755
- Sahajpal N, Mondal A, Ananth S, et al. Clinical utility of combined optical genome mapping and 523-gene next generation sequencing panel for comprehensive evaluation of myeloid cancers. medRxiv. 2022 Jan 17. doi: https://doi.org/10.1101/2022.01.15.22269355
- Sahajpal NS, Mondal AK, Tvrdik T, et al. Clinical Validation and Diagnostic Utility of Optical Genome Mapping for Enhanced Cytogenomic Analysis of Hematological Neoplasms [published online ahead of print, 2022 Oct 17]. J Mol Diagn. 2022;S1525-1578(22)00290-2. doi:10.1016/j.jmoldx.2022.09.009
- Tsui SP, Ip HW, Saw NY, et al. Redefining prognostication of de novo cytogenetically normal acute myeloid leukemia in young adults. Blood Cancer J. 2020;10(10):104. Published 2020 Oct 19. doi:10.1038/s41408-020-00373-4
- Walker A, Marcucci G. Molecular prognostic factors in cytogenetically normal acute myeloid leukemia. Expert Rev Hematol. 2012;5(5):547-558. doi:10.1586/ehm.12.45
- Graessner H, Zurek B, Hoischen A, Beltran S. Solving the unsolved rare diseases in Europe. Eur J Hum Genet. 2021;29(9):1319-1320. doi:10.1038/s41431-021-00924-8
- Yang H, Garcia-Manero G, Sasaki K, et al. High-resolution structural variant profiling of myelodysplastic syndromes by optical genome mapping uncovers cryptic aberrations of prognostic and therapeutic significance. Leukemia. 2022;36(9):2306-2316. doi:10.1038/s41375-022-01652-8
- Ebert P, Audano PA, Zhu Q, et al. Haplotype-resolved diverse human genomes and integrated analysis of structural variation. Science. 2021;372(6537):eabf7117. doi:10.1126/science.abf7117


