Blog
Unveiling the Power of Optical Genome Mapping: A Case Study on Cryptic Balanced Translocations
Key Takeaways
- OGM identified a cryptic balanced translocation, t(5; 6)(p15.31; p25.1), that was undetected by standard-of-care methods
- The study highlights OGM’s ability to uncover the genetic etiology behind abnormal fertility history and multiple anomalies across generations.
- OGM’s high-resolution, genome-wide, and unbiased approach facilitates the detection of structural variants with greater efficiency than conventional methods.
- The peer-reviewed study supports the indispensable role of OGM in constitutional genetics, particularly in post-natal scenarios.

In the intricate dance of genomics, where every twist and turn of DNA can lead to profound revelations or confounding mysteries, Optical Genome Mapping (OGM) has emerged as a beacon of clarity. A recent publication in Frontiers in Genetics by Xie et al. (2023) titled “Combination of trio-based whole exome sequencing and optical genome mapping reveals a cryptic balanced translocation that causes unbalanced chromosomal rearrangements in a family with multiple anomalies” underscores the efficacy of OGM in detecting complex genomic rearrangements that traditional methods may miss. The study was conducted by a team of researchers from the Ningbo Women and Children’s Hospital in Zhejiang, China.
The Case Study:
The publication presents a compelling case study of an 8-year-old girl with multiple anomalies and her family, who were subjected to trio-based whole exome sequencing (Trio-WES) and OGM. The proband exhibited conditions such as glaucoma, corneal opacities, and intellectual disability, among others. She was the focal point of the investigation. To construct a comprehensive genetic profile, the researchers extended their analysis to include six family members across three generations. The proband’s immediate family—her parents and sibling—underwent trio-based whole exome sequencing (Trio-WES) to identify any de novo mutations or inherited variants that could elucidate the proband’s condition. In parallel, the proband, her mother, and maternal grandmother were subjected to optical genome mapping (OGM). This method was particularly instrumental in uncovering structural variants and chromosomal rearrangements, providing a broader genomic context to the variations detected by Trio-WES. The grandmother’s inclusion was critical, as her genetic profile held the key to understanding the inherited nature of the genomic alterations. By employing both Trio-WES and OGM, the researchers could cross-validate their findings, ensuring a robust and comprehensive genetic analysis. This dual-approach methodology not only facilitated the discovery of a cryptic balanced translocation but also painted a detailed picture of the genomic landscape that contributed to the proband’s phenotype.
The genetic analysis revealed a heterozygous duplication in the 5p15.33–5p15.32 region of chromosome 5 and a heterozygous deletion in the 6p25.3–6p25.1 region of chromosome 6, inherited from the proband’s mother. The grandmother of the proband was found to carry a cryptic balanced translocation, t(5; 6)(p15.31; p25.1) (Figure 2, from Xie et al.), which led to unbalanced rearrangements in subsequent generations.
The Transformative Impact of OGM on Clinical Research Assays
OGM has been making waves in the realm of clinical research, particularly in the study of constitutional genetics. The ability of OGM to detect structural variants (SVs) with high precision is revolutionizing how researchers approach genomic analysis. Unlike traditional cytogenetic methods such as karyotyping, FISH, and microarrays, OGM provides a comprehensive view of the genome, enabling the detection of all classes of structural and numerical variants in a single assay. This not only maximizes the detection of key pathogenic variants but also simplifies laboratory workflows, driving efficiencies in cost and time to results.
OGM Versus Classical Methods
The case study by Xie et al. (2023) serves as a benchmark for comparing OGM with classical method assays. While classical method assays have been the cornerstone of genetic analysis for decades, they come with limitations, particularly in their resolution and scope. OGM, on the other hand, analyzes ultra-high molecular weight DNA, offering a genome-wide, high-resolution, unbiased, and digital approach. This allows for the detection of complex rearrangements, such as the cryptic balanced translocation t(5; 6)(p15.31; p25.1) in the proband’s grandmother, which would likely be missed by classical method analysis.
OGM’s Application in Various Genetic Conditions
OGM’s utility extends beyond the detection of balanced translocations. It has proven to be a valuable tool in the study of a wide range of genetic conditions, including but not limited to post-natal anomalies, constitutional genetics, and fertility-related disorders. The technology’s high-throughput nature and comprehensive analysis capabilities make it an ideal choice for clinical research assays, particularly in cases where traditional methods fall short.
Advancing Understanding of Complex Diseases
The integration of OGM into genetic research is paving the way for a deeper understanding of complex diseases. By providing a more complete picture of the genome, OGM facilitates the discovery of novel SVs and the elucidation of their roles in disease pathogenesis. This is particularly important in the field of constitutional genetics, where the detection of SVs can have significant implications for post-natal health and development.
Conclusion
The study by Xie et al. (2023) is a clear indication of the strides being made in genomic research, with OGM at the forefront. The technology’s ability to detect structural variants with unprecedented resolution and scope is transforming the landscape of clinical research assays. As we continue to harness the power of OGM, we can expect to see significant advancements in our ability to diagnose, understand, and ultimately treat complex genetic disorders.
Final Thoughts
As we embrace the advancements in genomic technologies, it is crucial for laboratories and research institutions to stay at the cutting edge of innovation. OGM represents a leap forward in our quest to unravel the complexities of the genome. For companies like Bionano, which are dedicated to providing genome analysis solutions, the future is bright. The journey of transforming the way the world sees the genome is well underway, and with each new discovery, we move closer to a world where the mysteries of the genome are no longer mysteries, but rather, keys to unlocking the full potential of human health and medicine.
References: Xie M, Xue J, Zhang Y, Zhou Y, Yu Q, Li H, and Li Q (2023). Combination of trio-based whole exome sequencing and optical genome mapping reveals a cryptic balanced translocation that causes unbalanced chromosomal rearrangements in a family with multiple anomalies. Front. Genet. 14:1248544. doi: 10.3389/fgene.2023.1248544
Key Figures from the article
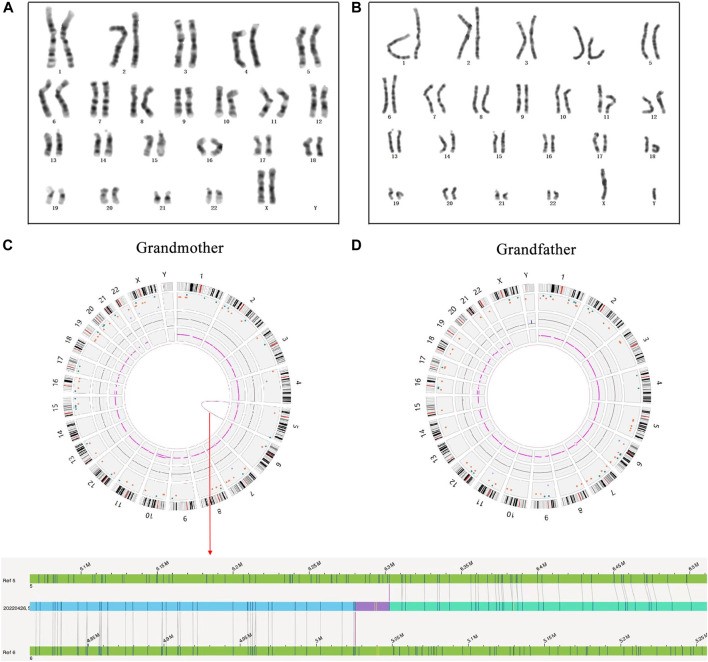
Figure 2. The results of high-resolution karyotype analysis and OGM analysis for the proband’s grandparents. (A) The description of grandmother’s karyotype was shown as 46,XX; (B) The description of grandfather’s karyotype was shown as 46,XY; (C) A cryptic BT between chromosomes 5 and 6 was uncovered in the grandmother by OGM: red arrow represents OGM analysis result; (D) No abnormity was found in the grandfather by OGM. (Xie et al., 2023)
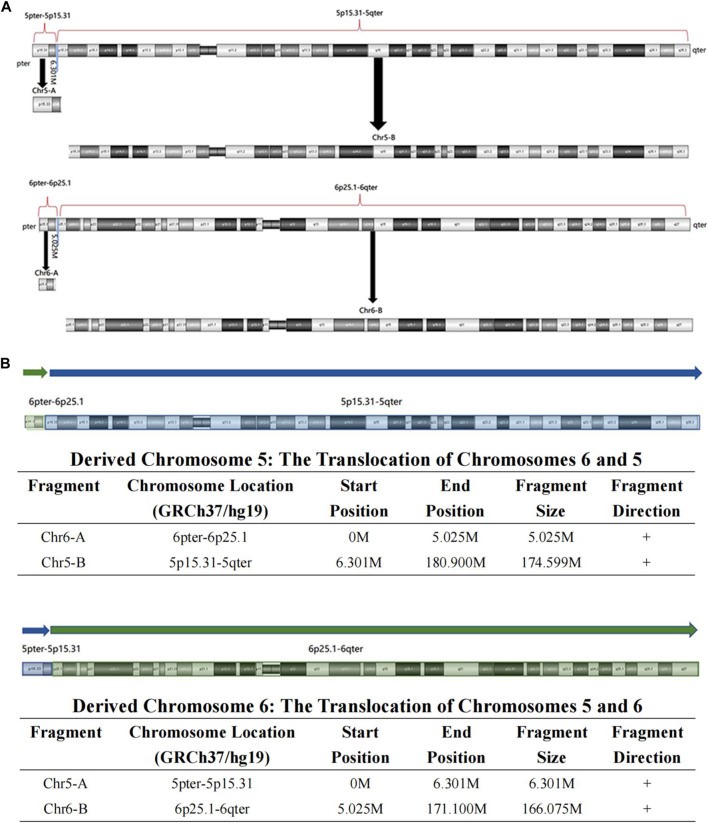
Figure 3. The visual BT results of OGM in the grandmother. (A) According to the breakpoint, the whole chromosome 5 was separated into Chr5-A and Chr5-B, and the whole chromosome 6 was separated into Chr6-A and Chr6-B; (B) The derived chromosome 5 comprises Chr6-A (6pter-6p25.1, 5.025 M) and Chr5-B (5p15.31-5qter, 174.599 M), and the derived chromosome 6 comprises Chr5-A (5pter-5p15.31, 6.301 M) and Chr6-B (6p25.1-6qter, 166.075 M). (Xie et al., 2023)




